Oxygen Evolution Reaction
References
- Vanadium-Incorporated CoP2 with Lattice Expansion for Highly Efficient Acidic Overall Water Splitting Angew. Chem.2022,134, e202116
- Uncovering the Effect of Lattice Strain and Oxygen Deficiency on Electrocatalytic Activity of Perovskite Cobaltite Thin Films Adv.Sci.2019, 6, 1801898
Mechanism of enhancement
Case I: Dope V into CoP2[Angew. Chem.2022,134, e202116]

the O XPS spectrum displays an obvious increased intensity of peak belonging to the adsorbed H2O (Figure 5h), suggesting the H2O adsorption ability is improved. Importantly, a new peak appears at 532.5 eV assigned to the oxygen vacancy,46 which often plays a significant role in boosting OER activity.47 Thus, we infer the reason that V promotes the activity and stability of CoP2 as follows: V possesses multiple valences. Particularly, the V4+ ion is extremely oxyphilic or hydrophilic because of its highly positive-charge and unfilled d-orbital,48 so that it facilitates the adsorption and dissociation of H2O to produce more hydroxyl groups, which is beneficial to subsequent OER (or HER) process. On the other hand, during OER electrolysis, as V changes its valence state from +4 to the highest +5, V5+ with enhanced oxidation properties could steer the valence state of Co element to higher and induce surface reconstitution to form a thicker acid-stable Co3O4 layer on the CoP2 surface, which in turn restrains the complete oxidation of CoP2, thereby enhancing the activity and resistance to corrosion ability in the acidic OER process.
Angew. Chem.2022,134, e202116
Case II: Coupling Effect Between Strain and Oxygen Defects and its Impact on the OER Activity of La0.7Sr0.3CoO3−δ (LSC)[Adv.Sci.2019, 6, 1801898]
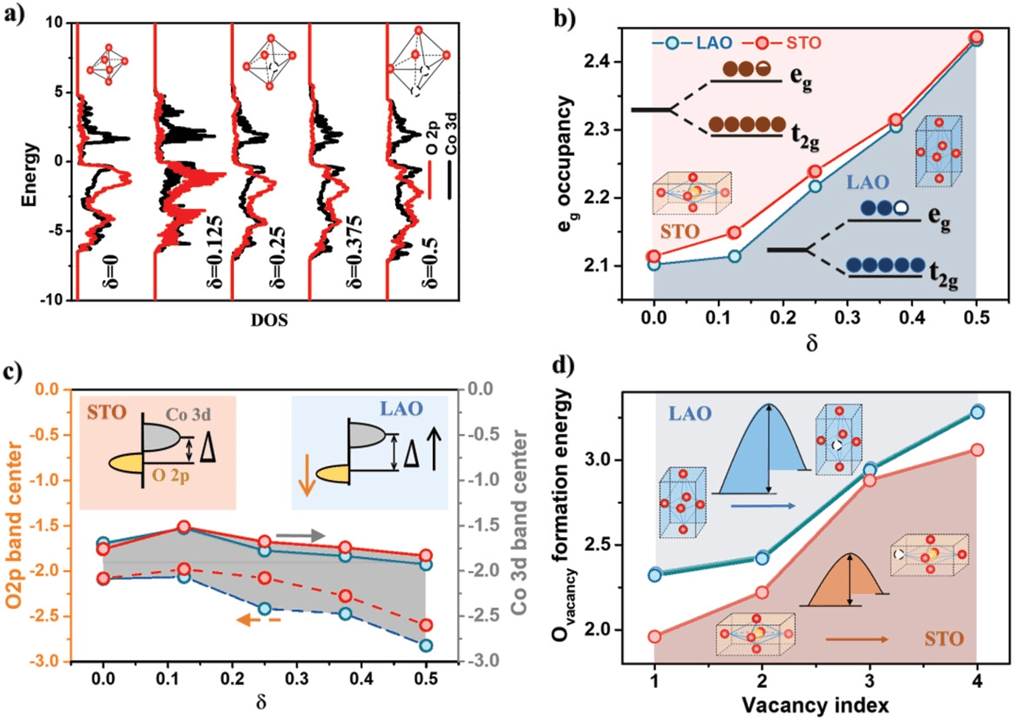
Two widely used electronic structure descriptors for OER activity were investigated systematically in this work: the occupancy of eg states (eg occupancy)[7, 8, 19] and the energy gap between O 2p and Co 3d band center (Δ)20 (Figure 5). It has been reported previously that the OER activity of perovskite oxides critically depend on its eg occupancy, with the optimal eg occupancy close to unity.[7, 8, 19] On the other hand, oxide catalysts with smaller Δ values were found to show higher electronic conductivity[20] and more facile charge transfer process during OER reaction.[20] Furthermore, previous work suggested that the OER on LSC can involve the exchange of lattice oxygen species,18, 21 and the increase in the covalency of metal–oxygen bonds, which can be quantified by a smaller energy gap between O 2p and Co 3d Δ or a higher O 2p-band center position relative to the Fermi level, can promote the participation of lattice-oxygen in OER and lead to higher OER activity of LSC.
We firstly compared the impacts of strain on the eg occupancy and Δ value of LSC for a δ value. As shown in Figure 5b, the eg occupancy for LSC/STO with tensile strain and LSC/LAO with compressive strain are quite close, with the eg occupancy for LSC/LAO to be slightly closer to the optimal value of unity. This result indicated that the LSC/LAO may show slightly more optimized bond strength to the reaction intermediates than the LSC/STO with the same δ, but such difference is not very significant. The Δ value, on the other hand, was found to be noticeably larger for the LSC/LAO than the LSC/STO with the same δ, suggesting lower electronic conductivity,[20] harder charge transfer process,[20] and less covalency of metal–oxygen bonds.18, 21 On the basis of the calculation results for eg occupancy and Δ value above, one should expect similar or even worse OER activity for the LSC/LAO than the LSC/STO for a given δ value, which is in contrast with the considerably better OER performance for LSC/LAO than LSC/STO we observed experimentally.
For both LSC films with tensile and compressive strain, our DFT results showed that the increase of δ lead to more electrons filled into the eg states (Figure 5b) and the increase of Δ value (Figure 5c). Such changes in electronic structure are likely to be the reason for the degraded OER activities for vacuum annealed LSC we observed in the electrochemical test.
As mentioned in the introduction section, lattice strain and oxygen defect chemistry are reported to be strongly coupled with each other.9 To understand such coupling effect for LSC, DFT calculations were carried out to determine the oxygen vacancy formation energy for the LSC/STO and the LSC/LAO using the strain value extracted from the HRXRD experiments. As shown in Figure 5d, the oxygen vacancy formation energy for the LSC/STO with tensile strain is considerably smaller than that for the LSC/LAO with compressive strain. These results are consistent with the higher O 2p band center for LSO/STO shown in Figure 5c, suggesting a smaller energy penalty for electrons to excite from O 2p band to either Co 3d band or near the Fermi level during oxygen vacancy formation.22 As a result of the smaller oxygen vacancy formation energy, the LSC/STO is likely to be equilibrium with more oxygen vacancies in the lattice than LSC/LAO under similar environments, leading to the larger δ value for LSC/STO as we observed in the HRXRD results. This conclusion is also supported by our XPS results which showed that LSC/STO exhibited more Co2+ than LSC/LAO after annealing, and is consistent with previous reports of Cai et al.[9] The lower δ value in LSC/LAO than that in LSC/STO were likely to result in a considerably smaller eg states occupancy and a similar or even smaller Δ for LSC/LAO than LSC/STO. Both factors can eventually lead to the better OER activity of the LSC/LAO.
In this work, we found that the creation of excessive oxygen vacancies in LSC leads to lower OER activity, attributed to more deviation of eg filling from unity and larger energy gap between O 2p and Co 3d band. Similar detrimental effects of oxygen vacancies on the OER and ORR activity of La0.6Ca0.4CoO3−δ were reported by Wu at el.[8] For CaMn0.75Nb0.25O3−δ, on the other hand, the presence of oxygen vacancies was reported to enhance the OER and ORR activity, attributed to optimized eg filling, stronger OH− adsorption, and improved electrical conductivity.[8] Xu et al. reported significantly improved OER activity of Co3O4 with abundant oxygen vacancies created by plasma treatment, and believed the enhanced performance was due to the improved electronic conductivity and higher active defects density.[8] All these results demonstrate that the effects of oxygen defects on the electrocatalytic activity of metal oxides depend critically on the material systems. It is critical to further investigate the role of oxygen defects for the rational design of high performance catalysts.
It is worth noting that we observed a decrease of surface Sr contents for both the LSC/STO and the LSC/LAO after OER test (Figure S8, Supporting Information), which is likely to be the reason for the activation process during the first few test cycles as shown in Figure S9 in the Supporting Information.23 Although previous works showed that surface chemistry can strongly impact the surface reaction kinetics of perovskite oxides,24 we did not observe noticeable difference in the surface composition between the LSC/STO and the LSC/LAO films. Therefore, we believe that the surface composition is not the major factor that leads to the different OER activity between the LSC/STO and the LSC/LAO. Furthermore, to understand the correlation among lattice strain, oxygen defects, and OER activity for LSC thin film, we calculated the eg occupancy (Figure 5b) and the Δ value (Figure 5c) as the function of strain and oxygen nonstoichiometry. There are other electronic structure descriptors for OER activity reported in literature.25 To survey all the electronic structure descriptors is beyond the scope of this manuscript. Our results, however, indeed have demonstrated the critical impact of strain–defect coupling effects on the electrocatalytic.
Adv.Sci.2019, 6, 1801898
Hydrogen Evolution Reaction
References
- Tailoring the d-Band Centers Enables Co4N Nanosheets To Be Highly Active for Hydrogen Evolution Catalysis Angew.Chem.Int.Ed.2018,57,5076–5080
Mechanism of enhancement
Case I: Dope V into Co4N
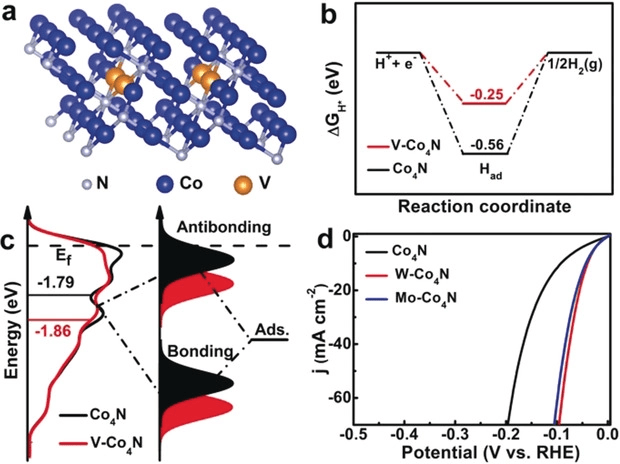
the electronic interaction could be considered as the coupling between the adsorbate valence states and the transition-metal d states, which gives rise to the formation of separated bonding and antibonding states. The bonding states are generally fully filled because they are far below the Fermi level (Ef), while the electron filling of the antibonding states depends on these energy states relative to the Fermi level and contributes to the bond strength. Since the antibonding states are always above the d states in terms of energy, the d band center model would be a good descriptor of the adsorbate–metal interaction. Impressively, the d-band center (Ed) of Co4N and V-Co4N relative to Ef are calculated to be −1.79 eV and −1.85 eV, respectively (Figure 4 c), which clearly illustrates that the d-band center is far away from the Fermi level after V doping. It also suggests the antibonding energy states are lowered and the interaction between the adsorbate and surface is weakened, which is in agreement with the UPS results, where the valence band is shifted away from the Fermi level after V doping. These studies provide the intrinsic elucidation that the downshift of the d-band center as the antibonding states are filled with more electrons after V doping decreases the adsorption energy of H and meanwhile facilitates the desorption of H from the catalyst surface for HER catalysis.
Angew.Chem.Int.Ed.2018,57,5076–5080

Leave a comment