Case I: Nitride
Example 1: Copper
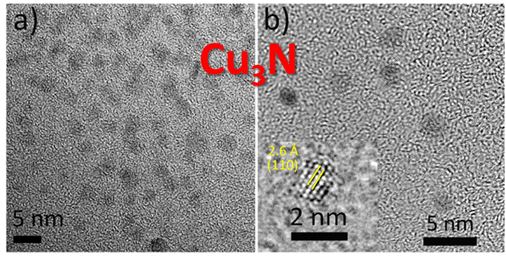
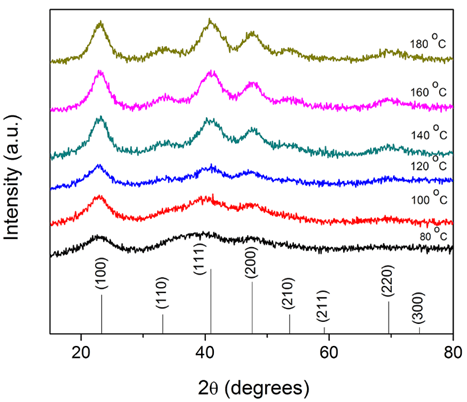
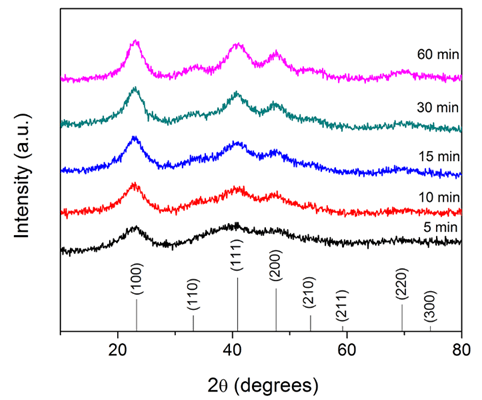
50 mg of Cu(OMe)2 was added into a 10 mL glass vial. Afterward 5 mL benzylamine was added and the vessel was sealed with a Teflon cap and taken out of the glovebox. The reaction vessel was transferred into a preheated oil bath set at 140 °C and held at that temperature for 15 min under vigorous magnetic stirring. During heating, after about 3 min, the color of the reaction solution changed from dark blue to red. Afterward, the reaction mixture was cooled down to room temperature. The product was precipitated by adding 40 mL of hexane and collected by centrifugation at 4000 rpm for 15 min. The product was washed two more times using 40 mL of hexane and finally dried under nitrogen flux. The yield of the reaction was >90% with respect to Cu(OMe)2. To obtain stable colloidal dispersions of Cu3N nanoparticles, the wet products obtained after washing were directly dispersed in chloroform, THF, and NMP using ultrasonication for 10 min.
Chem. Mater. 2015, 27, 8282−8288
Scheme 1. Proposed Reaction Mechanism for the Main Processes Involved in the Formation of Cu3 N Nanoparticles
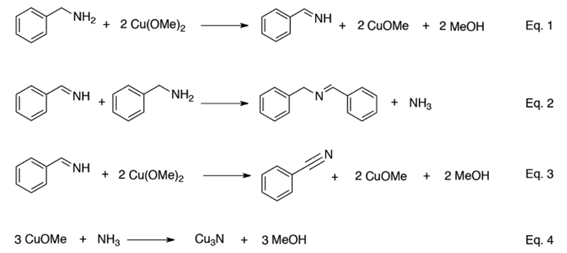
In the first step, a benzylamine molecule reacts with two Cu(II) ions to form methanol, Cu(I) and benzenemethanimine (eq 1, Scheme 1), out of which methanol is observed in the GC with retention time of 4.65 min. During this step, the high Lewis basicity of benzylamine effectively solvates the Cu(II) ions and the high oxidation efficiency of Cu(II) ions is responsible for oxidation of benzylamine to benzenemethanimine. In the present case, Cu(OMe)2 offers a quite exceptional reactivity since Cu(II) salts usually provide stable complexes with amines. For example, we observed that Cu(II) chloride forms a blue solution with benzylamine and fails to produce Cu3N under the same reaction conditions. The in situ produced benzenemethanimine reacts further with benzylamine to form N-benzylidenebenzylamine and ammonia (eq 2, Scheme 1). Garnweitner et al. also reported the formation of N-benzylidenebenzylamine during the solvothermal treatment of titanium isopropoxide in benzylamine. The N-benzylidenebenzylamine appears in considerable amount in the GC with retention time of 21.70 min. Furthermore, we observed trace quantities of benzonitrile in the GC with retention time of 12.32 min. The benzonitrile formation can be explained as a further molecule of benzenemethanimine oxidizes to benzonitrile by reaction with Cu(II) ions (eq 3, Scheme 1). However, the Cu(II) ion concentration should be large enough to prevent competitive addition of benzylamine to benzenemethanimine (eq 2). During the last step, the in situ produced ammonia immediately reacts with CuOMe to form Cu3N and methanol (eq 4, Scheme 1). The strong basicity of methoxide ions provides the driving force for the nitridation reaction.

Leave a comment