Case I: Metal acetylacetonate
Example 1: Nickel
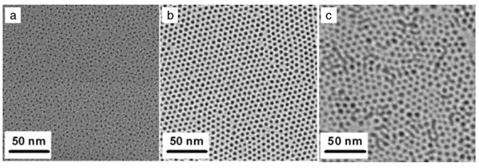
First, the Ni±oleylamine complexwas prepared by reacting 0.52 g of nickel(acetylacetonate)2[Ni(acac)2, Aldrich Chemical Co., 95%] and 2 mL of oleyla-mine (Aldrich Chemical Co., technical grade, 80%) at 100Cunder an Ar atmosphere. The resulting metal-complex solu-tion was injected into 5 g of triphenylphosphine (TPP, AldrichChemical Co., 99%) at 215C. The color of the solutionchanged from green to black, indicating that colloidal nano-particles were generated. The black solution was aged at200 Cfor 30 min, and was then cooled to room temperature.The nanoparticles were precipitated by adding excess ethanolto the solution.
Adv.Mater.2005,17, No. 4, February 23

Example 2: Nickel
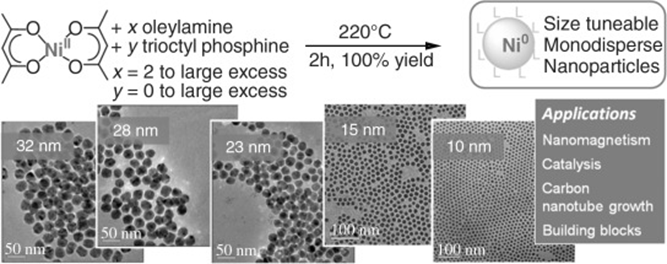
In a typical experiment, oleylamine (4 equiv, 3.12×10−3 mol, 834 mg) was introduced in a Schlenk tube and degassed under vacuum. [Ni(acac)2] (1 equiv, 7.8×10−4 mol, 200 mg) was then added to the oleylamine. The mixture was heated to the desired temperature by using an oil bath. As 215 °C, the solution turned black. After 10 min to 2 h of heating, the solution was cooled. The nanoparticles were collected by centrifugation, and the supernatant was stored for further analysis. The reaction was conducted similarly by using [Pd(acac)2]. Oleylamine (4 equiv, 3.12×10−3 mol, 834 mg) was introduced into a Schlenk tube and degassed under vacuum. [Pd(acac)2] (1 equiv, 7.8×10−4 mol, 237 mg) was then added to the oleylamine. The mixture was heated to the desired temperature by using an oil bath. As 180 °C, the solution turned black. After 10 min of heating, the solution was cooled.
Chem. Eur. J.2012,18, 14165 – 14173

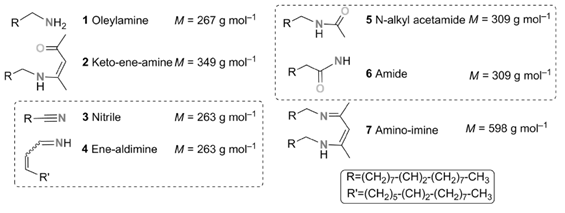
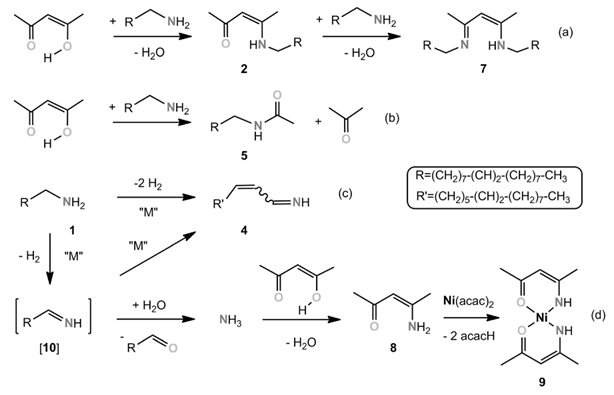
At this point, a radical pathway could be definitively excluded for two reasons: 1) radical formation by oxidation of amines has been reported in the literature, but only in the presence of a strong oxidizing agent, generated NiIII complexes.38 Indeed, the standard redox potential for the couple NR3+./NR3 is between 1.0 and 1.9 V, whereas the Ni(+2)/Ni one is only −0.26 V.39 Even taking into consideration the variation from standard potentials under the present conditions, NiII is clearly not a strong enough oxidizing agent to oxidize the oleylamine through a radical pathway; and 2) experimentally, if ever the RNH2+. radical were to form, it would mean that strong oxidizing conditions were encountered and the nitrile byproduct 3 should form in the medium, which was not the case.40 Moreover, the radical itself would break and/or dimerize and give corresponding byproducts in significant amounts, since 2 equiv of it would be formed during the reaction.

Altogether, the mechanism presented here is the most favorable that could be computed. It fully accounts for all the features observed experimentally: the need for at least 3 equiv of oleylamine (4 equiv are more favorable in solvent-assisted processes), the production of water in the early stages of heating (100–120 °C), the need for a more severe temperature (at 180 °C) for Pd reduction in the last step, and the production of the keto-ene-amine 2 and the primary aldimine 10. Moreover, the fast nucleation process of nanoparticles, which corresponds to a swift color change to black, correlates well with the formation of a poorly stabilized Pd0 complex as a result of the reduction step. Note also that the formation of a nitrile, by means of H2 elimination from the imine, was probed by calculations, but a reasonable path could not be obtained.
Example 3: Metal Oxide
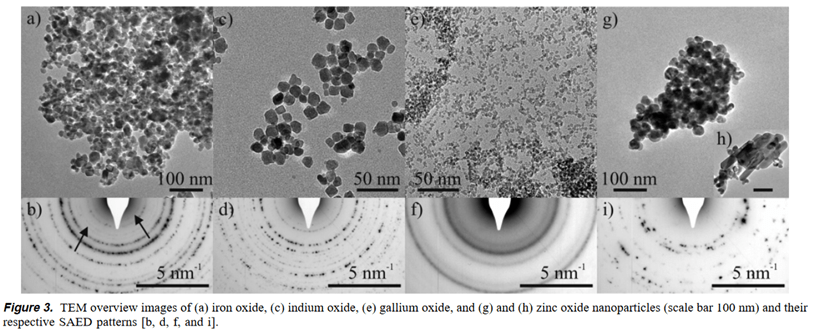
In a typical synthesis of nanoparticles, 1.0 g of Fe(acac) 3 (2.81 mmol), 0.5 g of In(acac) 3 (1.21 mmol), 0.5 g of Ga(acac)3 (1.36 mmol), or 0.5 g of Zn(acac) 2‚xH 2O (1.90 mmol), respectively, was added to 20 mL of benzylamine. The reaction mixture was transferred into a Teflon cup of 45 mL inner volume, slid into a steel autoclave, and carefully sealed. The autoclave was taken out of the glovebox and heated in a furnace at 200 °C for 2 days. The resulting milky suspensions were centrifuged, and the precipitates thoroughly washed with ethanol and dichloromethane and subsequently dried in air at 60 °C
J. AM. CHEM. SOC. 2005, 127, 5608-5612
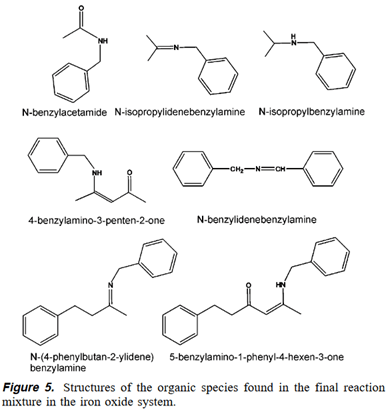
- N-Benzylidenebenzylamine is formed by dehy- drogenative oxidation of benzylamine and thus explains the formation of magnetite
- The presence of 4-benzylamino-3-penten-2-one, on the other hand, proves that not all acetylacetone is cleaved to form the amide
- N-(4-Phenylbutan-2-ylidene)benzylamine and 5-benzylamino-1-phenyl-4-hexen-3-one are formed in an analogous fashion.

The reaction starts with the nucleophilic attack of benzylamine on one carbonyl center of the acetylacetonate (1). In contrast to the aminolysis, the C-C bond is not broken (2). The oxygen is released in the form of a hydroxyl group, (3*), concurrently leading to 4-benzylamino-3-penten-2-one.
The enolate ligand (formed during the main reaction, see Scheme 1) now acts as a nucleophile. As benzylamine is a good nucleophile itself, an attack of the amine is rather improbable. It is more likely that an amide is attacked (4*), as the cleavage of benzyl groups from amide nitrogen is known to proceed under reasonably mild conditions. 29 This reaction would lead to the release of acetamide
Case II: Metal dithiocarbamate complexes
[Zn(S 2 CNi Bu2 ) 2 ] (5 mM) was added to oleylamine (20 mL) in a three-neck round bottom flask attached to a condenser and evacuated and refllled with nitrogen repeatedly for 15 minutes. The solution was heated to 230 C and held there for 1 h. The mixture was allowed to cool to room temperature slowly, where upon methanol (80 mL) was added with stirring. The mixture was centrifuged and then the solution decanted leaving behind the resultant nanoparticles.
Nanoscale Adv., 2020, 2, 798


Leave a comment