References
- Photoelectrochemical Nitrogen Reduction to Ammonia on Cupric and Cuprous Oxide Photocathodes: ACS Energy Lett. 2020, 5, 6, 1834–1839
ElectroChemistry (EC) vs. PhotoElectroChemistry (PEC)
In electrochemical N2 reduction, N2 reduction at the cathode is paired with water oxidation at the anode (Scheme 1a). The overall reaction is thermodynamically uphill, and therefore, an electrochemical N2 reduction cell requires an electrical energy input as in the case of an electrochemical water splitting cell. A photoelectrochemical cell can be constructed if the cathode is replaced with a photocathode that can utilize solar energy to provide a photovoltage. This can partially or completely eliminate the electrical energy input required for N2 reduction depending on the Fermi level of the photocathode under illumination (Scheme 1b).
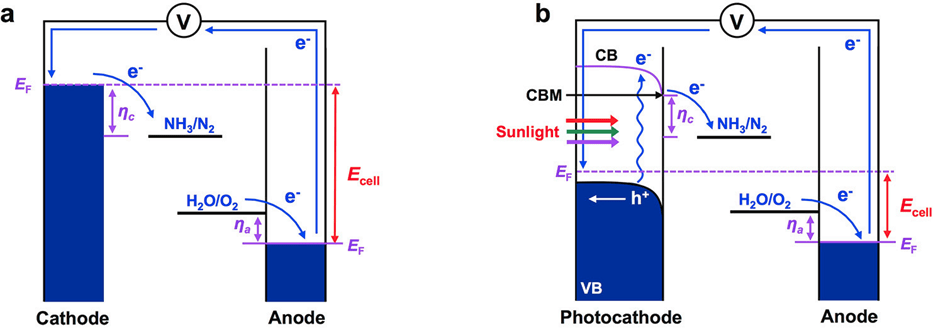
Figure. Comparison of (a) Electrochemical and (b) Photoelectrochemical N2 Reduction, Illustrating Why the Use of a Photocathode Can Decrease the Overall Cell Voltage Needed to Operate the N2 Reduction Cella [ACS Energy Lett. 2020, 5, 6, 1834–1839]
aEcell is the voltage required to operate the cell, EF the Fermi level, CB the conduction band, VB the valence band, CBM the conduction band minimum, ηc the cathodic overpotential, and ηa the anodic overpotential.

Leave a comment